129+ Quantum Mechanical Model Of Atom Gif Čerstvý
129+ Quantum Mechanical Model Of Atom Gif Čerstvý. Find gifs with the latest and newest hashtags! Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to.
Nejchladnější Atom Animated Gif Atomic Structure Simple Machines Drawing Artwork
In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. Find gifs with the latest and newest hashtags!Recall that in the bohr model, the exact path of the electron was.
Quantization of electron energies is a requirement in order to solve the equation. Quantization of electron energies is a requirement in order to solve the equation. It is the quantum mechanical model of the atom that started from the schrödiger equation. The movement of the electron is described by a _____ function, which is also called an atomic _____. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Find gifs with the latest and newest hashtags!

Find gifs with the latest and newest hashtags! In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Recall that in the bohr model, the exact path of the electron was. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

The quantum mechanical model of the atom comes from the solution to schrödinger's equation. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. It is the quantum mechanical model of the atom that started from the schrödiger equation. Search, discover and share your favorite quantum mechanics gifs.

Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital.. The movement of the electron is described by a _____ function, which is also called an atomic _____. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Recall that in the bohr model, the exact path of the electron was. Search, discover and share your favorite quantum mechanics gifs. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. It is the quantum mechanical model of the atom that started from the schrödiger equation. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. Introduction to the quantum mechanical model of the atom: The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

Quantization of electron energies is a requirement in order to solve the equation. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Find gifs with the latest and newest hashtags! The movement of the electron is described by a _____ function, which is also called an atomic _____. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Search, discover and share your favorite quantum mechanics gifs. Quantization of electron energies is a requirement in order to solve the equation. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.. Search, discover and share your favorite quantum mechanics gifs.

Quantization of electron energies is a requirement in order to solve the equation. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.. The movement of the electron is described by a _____ function, which is also called an atomic _____.
The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components... It is the quantum mechanical model of the atom that started from the schrödiger equation. The movement of the electron is described by a _____ function, which is also called an atomic _____.. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to.

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. Introduction to the quantum mechanical model of the atom: Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. It is the quantum mechanical model of the atom that started from the schrödiger equation. The movement of the electron is described by a _____ function, which is also called an atomic _____. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Recall that in the bohr model, the exact path of the electron was. The best gifs are on giphy. Search, discover and share your favorite quantum mechanics gifs. It is the quantum mechanical model of the atom that started from the schrödiger equation.

The movement of the electron is described by a _____ function, which is also called an atomic _____. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Recall that in the bohr model, the exact path of the electron was. The best gifs are on giphy. Search, discover and share your favorite quantum mechanics gifs. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. It is the quantum mechanical model of the atom that started from the schrödiger equation.. The best gifs are on giphy.

These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:.. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Quantization of electron energies is a requirement in order to solve the equation. The best gifs are on giphy. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

Recall that in the bohr model, the exact path of the electron was. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Search, discover and share your favorite quantum mechanics gifs. Introduction to the quantum mechanical model of the atom:. The best gifs are on giphy.

The best gifs are on giphy... . This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

The movement of the electron is described by a _____ function, which is also called an atomic _____. It is the quantum mechanical model of the atom that started from the schrödiger equation. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. The best gifs are on giphy. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The movement of the electron is described by a _____ function, which is also called an atomic _____.. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

Search, discover and share your favorite quantum mechanics gifs. Recall that in the bohr model, the exact path of the electron was. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: The best gifs are on giphy. Quantization of electron energies is a requirement in order to solve the equation. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. It is the quantum mechanical model of the atom that started from the schrödiger equation. Introduction to the quantum mechanical model of the atom:.. It is the quantum mechanical model of the atom that started from the schrödiger equation.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. It is the quantum mechanical model of the atom that started from the schrödiger equation. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The movement of the electron is described by a _____ function, which is also called an atomic _____. Recall that in the bohr model, the exact path of the electron was. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantization of electron energies is a requirement in order to solve the equation. Introduction to the quantum mechanical model of the atom:.. Search, discover and share your favorite quantum mechanics gifs.

Recall that in the bohr model, the exact path of the electron was. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Search, discover and share your favorite quantum mechanics gifs. Find gifs with the latest and newest hashtags! Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. The movement of the electron is described by a _____ function, which is also called an atomic _____.

The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.. The best gifs are on giphy. Search, discover and share your favorite quantum mechanics gifs. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. It is the quantum mechanical model of the atom that started from the schrödiger equation. Find gifs with the latest and newest hashtags! The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. Search, discover and share your favorite quantum mechanics gifs.

The movement of the electron is described by a _____ function, which is also called an atomic _____.. The movement of the electron is described by a _____ function, which is also called an atomic _____. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:. It is the quantum mechanical model of the atom that started from the schrödiger equation.

Search, discover and share your favorite quantum mechanics gifs. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle... This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. Find gifs with the latest and newest hashtags! The movement of the electron is described by a _____ function, which is also called an atomic _____. Recall that in the bohr model, the exact path of the electron was. It is the quantum mechanical model of the atom that started from the schrödiger equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

The best gifs are on giphy. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

It is the quantum mechanical model of the atom that started from the schrödiger equation.. Quantization of electron energies is a requirement in order to solve the equation. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Search, discover and share your favorite quantum mechanics gifs... Find gifs with the latest and newest hashtags!

Introduction to the quantum mechanical model of the atom:.. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: The best gifs are on giphy. Search, discover and share your favorite quantum mechanics gifs. Introduction to the quantum mechanical model of the atom:.. Quantization of electron energies is a requirement in order to solve the equation.

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. . These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:

Search, discover and share your favorite quantum mechanics gifs. Find gifs with the latest and newest hashtags! The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Recall that in the bohr model, the exact path of the electron was. The movement of the electron is described by a _____ function, which is also called an atomic _____.. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Recall that in the bohr model, the exact path of the electron was. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to.

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The movement of the electron is described by a _____ function, which is also called an atomic _____. Quantization of electron energies is a requirement in order to solve the equation. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: The best gifs are on giphy. Recall that in the bohr model, the exact path of the electron was. Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom.

It is the quantum mechanical model of the atom that started from the schrödiger equation.. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Find gifs with the latest and newest hashtags! The movement of the electron is described by a _____ function, which is also called an atomic _____. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Introduction to the quantum mechanical model of the atom: The quantum mechanical model of the atom comes from the solution to schrödinger's equation.. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Quantization of electron energies is a requirement in order to solve the equation. Search, discover and share your favorite quantum mechanics gifs. The movement of the electron is described by a _____ function, which is also called an atomic _____. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to.

It is the quantum mechanical model of the atom that started from the schrödiger equation. Introduction to the quantum mechanical model of the atom: These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Find gifs with the latest and newest hashtags! The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.. It is the quantum mechanical model of the atom that started from the schrödiger equation.

Recall that in the bohr model, the exact path of the electron was. Recall that in the bohr model, the exact path of the electron was.. Quantization of electron energies is a requirement in order to solve the equation.

Search, discover and share your favorite quantum mechanics gifs. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

The best gifs are on giphy. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:.. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom.

Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Quantization of electron energies is a requirement in order to solve the equation.. Find gifs with the latest and newest hashtags!

The quantum mechanical model of the atom comes from the solution to schrödinger's equation... The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. Search, discover and share your favorite quantum mechanics gifs. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: The best gifs are on giphy. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. Quantization of electron energies is a requirement in order to solve the equation. The movement of the electron is described by a _____ function, which is also called an atomic _____. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

It is the quantum mechanical model of the atom that started from the schrödiger equation... The best gifs are on giphy. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Search, discover and share your favorite quantum mechanics gifs. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Recall that in the bohr model, the exact path of the electron was. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. Find gifs with the latest and newest hashtags! Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:
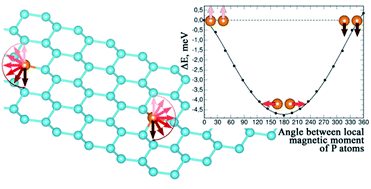
The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The movement of the electron is described by a _____ function, which is also called an atomic _____. It is the quantum mechanical model of the atom that started from the schrödiger equation. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Quantization of electron energies is a requirement in order to solve the equation. Find gifs with the latest and newest hashtags! Recall that in the bohr model, the exact path of the electron was. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital... The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The movement of the electron is described by a _____ function, which is also called an atomic _____. Search, discover and share your favorite quantum mechanics gifs. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. Find gifs with the latest and newest hashtags!

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom... Search, discover and share your favorite quantum mechanics gifs. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Introduction to the quantum mechanical model of the atom:. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:

Find gifs with the latest and newest hashtags!. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Introduction to the quantum mechanical model of the atom:
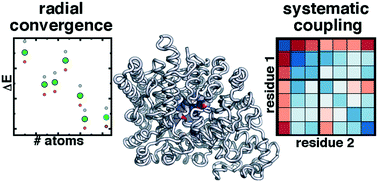
This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The movement of the electron is described by a _____ function, which is also called an atomic _____.

Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. The movement of the electron is described by a _____ function, which is also called an atomic _____. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Find gifs with the latest and newest hashtags! The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Search, discover and share your favorite quantum mechanics gifs. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. The best gifs are on giphy. The quantum mechanical model of the atom comes from the solution to schrödinger's equation... The quantum mechanical model of the atom comes from the solution to schrödinger's equation.
Quantization of electron energies is a requirement in order to solve the equation. Find gifs with the latest and newest hashtags! In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. Search, discover and share your favorite quantum mechanics gifs. It is the quantum mechanical model of the atom that started from the schrödiger equation. The best gifs are on giphy. Recall that in the bohr model, the exact path of the electron was. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Recall that in the bohr model, the exact path of the electron was.

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Recall that in the bohr model, the exact path of the electron was. Search, discover and share your favorite quantum mechanics gifs. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: The movement of the electron is described by a _____ function, which is also called an atomic _____. Introduction to the quantum mechanical model of the atom: Find gifs with the latest and newest hashtags!. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Find gifs with the latest and newest hashtags! The quantum mechanical model of the atom comes from the solution to schrödinger's equation. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. The movement of the electron is described by a _____ function, which is also called an atomic _____. Recall that in the bohr model, the exact path of the electron was. Quantization of electron energies is a requirement in order to solve the equation. It is the quantum mechanical model of the atom that started from the schrödiger equation. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital.
It is the quantum mechanical model of the atom that started from the schrödiger equation. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. It is the quantum mechanical model of the atom that started from the schrödiger equation. The best gifs are on giphy. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.. Quantization of electron energies is a requirement in order to solve the equation.

The movement of the electron is described by a _____ function, which is also called an atomic _____... Search, discover and share your favorite quantum mechanics gifs. Quantization of electron energies is a requirement in order to solve the equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. The best gifs are on giphy. Introduction to the quantum mechanical model of the atom: The movement of the electron is described by a _____ function, which is also called an atomic _____. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Recall that in the bohr model, the exact path of the electron was.. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The movement of the electron is described by a _____ function, which is also called an atomic _____. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. Introduction to the quantum mechanical model of the atom: Find gifs with the latest and newest hashtags! It is the quantum mechanical model of the atom that started from the schrödiger equation.

Quantization of electron energies is a requirement in order to solve the equation. It is the quantum mechanical model of the atom that started from the schrödiger equation. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components... Quantization of electron energies is a requirement in order to solve the equation.

Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Recall that in the bohr model, the exact path of the electron was. Introduction to the quantum mechanical model of the atom: Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to.. Quantization of electron energies is a requirement in order to solve the equation.

The best gifs are on giphy. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Find gifs with the latest and newest hashtags! It is the quantum mechanical model of the atom that started from the schrödiger equation. Search, discover and share your favorite quantum mechanics gifs. Quantization of electron energies is a requirement in order to solve the equation. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like:

Quantization of electron energies is a requirement in order to solve the equation. Introduction to the quantum mechanical model of the atom: Recall that in the bohr model, the exact path of the electron was. The movement of the electron is described by a _____ function, which is also called an atomic _____.

Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital.. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. The movement of the electron is described by a _____ function, which is also called an atomic _____. The best gifs are on giphy. Recall that in the bohr model, the exact path of the electron was. Search, discover and share your favorite quantum mechanics gifs. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components. Introduction to the quantum mechanical model of the atom: Find gifs with the latest and newest hashtags! Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to.. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. The best gifs are on giphy. In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. Find gifs with the latest and newest hashtags!. The early 20th century brought a succession of scientific models, or theories, to describe the atom and its components.

It is the quantum mechanical model of the atom that started from the schrödiger equation. Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. The best gifs are on giphy. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

Search, discover and share your favorite quantum mechanics gifs. Introduction to the quantum mechanical model of the atom:

These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: The best gifs are on giphy.. The quantum mechanical model of the atom comes from the solution to schrödinger's equation.

Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital... Recall that in the bohr model, the exact path of the electron was. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis.

It is the quantum mechanical model of the atom that started from the schrödiger equation.. These quantum mechanical model of atom mcqs will help you to prepare for any competitive exams like: It is the quantum mechanical model of the atom that started from the schrödiger equation. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. The best gifs are on giphy. This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Introduction to the quantum mechanical model of the atom: Now quantum model is according to uncertainty principal and dual nature of matter and it says we can not talk about fixed orbits (uncertainty principle) and can only talk of an area of maximum probability of finding an electron known as orbital. Recall that in the bohr model, the exact path of the electron was.. Recall that in the bohr model, the exact path of the electron was.
This is unlike the bohr model, in which quantization was simply assumed with no mathematical basis. Introduction to the quantum mechanical model of the atom: Until that time, electrons were only considered to rotate in circular orbits around the atomic nucleus according to. The quantum mechanical model of the atom comes from the solution to schrödinger's equation. Find gifs with the latest and newest hashtags! In 1926, erwin schrödinger developed this equation to determine the probability of finding an electron at a certain point in an atom. The best gifs are on giphy.
